
Founded in 1895 as Peiyang University, Tianjin University is the oldest institute of higher education in China, and pioneered the development of modern Chinese education.



Founded in 1895 as Peiyang University, Tianjin University is the oldest institute of higher education in China, and pioneered the development of modern Chinese education.

Tianjin University adheres to the mission of training high caliber talents with innovative abilities, and has had considerable success in the training of high-level specialists in various fields.

Tianjin University constantly refines and optimizes the structure of academic disciplines, and has set up 61 undergraduate programs, 35 master degree programs, and 27 doctoral degree programs.

A rich campus life awaits all those who come to Tianjin University. An integral part of our mission to train talented graduates is providing a fertile environment for students to develope their social, cultural, sporting and artistic lives.

It is reported that worldwide 60 million people suffer from epilepsy. Scientists have been dedicated to discovering the causes and treatment for this disease.
Mutations in potassium cation–chloride cotransporter (KCC), a protein that plays important roles in human cell volume regulation, salt reabsorption and GABAergic modulation by transporting ions across the plasma membrane may be one of the causes that leads to epilepsy and other genetic disorders of the human nervous system.
On October 25, 2019, Dr. Si Liu from Tianjin University’s School of Life Sciences, together with her collaborators published an article entitled “Cryo-EM Structures of the Human Cation-Chloride Cotransporter KCC1” in Science online,one of the most renowned academic journals in the world. The paper made a great breakthrough in presenting the structure of KCC1, providing a framework for interpreting disease-related mutations of KCCs and promoting drug design accordingly.
The researchers succeeded in presenting three cryo–electron microscopy (cryo-EM) structures of human KCC1 at a 2.9- to 3.5-angstrom resolution. “We found that KCC1 exists as a dimer, its extracellular and transmembrane domains both involved in dimerization.” Liu introduced. Through structural and functional analyses and computational studies, Liu and her collaborators also revealed one potassium site and two chloride sites in KCC1, which are all required for the ion transport activity. “The KCC1 structures allow us to model a potential ion transport mechanism in KCCs and provide a blueprint for drug design,” said Liu.
The research was conducted under the joint efforts of five teams, namely Prof. Jiangtao Guo’s team and Prof. Jingyuan Li’s team from Zhejiang University, Prof. Xiaochen Bai’s team from UT Southwestern Medical Center, USA, Prof. Sheng Ye’s team from Tianjin University and Professor Eric Delpire’s team from Vanderbilt University, USA. Si Liu from Tianjin University, Shenghai Chang and Binming Han both from Zhejiang University share the honor of the first writers together in the publishedScience article.
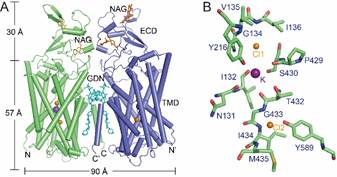
Figure A: Overall structure of human KCC1 exists as a dimer; Figure B:A zoom-in viewshowing the binding sites of K+and Cl-in KCC1
By Eva Yin
Editor: Doris Harrington